Ultrasound of the Week #039
Case:
A 70 year old male with a PMH of hypertension presented with 2 weeks of feeling generally unwell, with epigastric pain radiating through to their back, 8kg of weight loss over 1 month and dizziness/breathlessness on standing. They reported feeling food did not go down easily and was on H.pylori eradication therapy from their GP.
Obs on arrival were: SpO2 95% (on 15L NRBM), BP 109/72, RR28
He was sent for a CTPA and on his return was found to be unresponsive with no palpable central pulse. Chest compressions were started and he then started to respond and a central pulse was felt.
A bedside echo was then performed by the Emergency Medicine Registrar with the below images:
Question: What does each view show?
[expand title=”Answer & Explanation:” tag=”h2″]
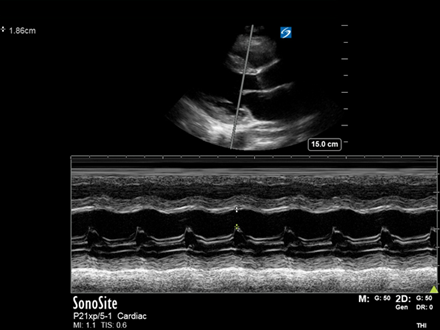
PLAX: The PLAX view shows a clearly small, hyperdynamic LV. The LV walls are seen to ‘kiss’ during systole and the AMVL is coming up to ‘slap’ the IVS, a quick ‘eyeball’ indicator of a good LVEF. A reduced LVIDd (LV Internal Diameter at end-diastole) is a great indicator of low LV preload(see USOTW 035 for details on measurement). The RV is dilated.
PSAX: The RV is clearly dilated, with an RV:LV ratio >1. Additionally there is flattening of the IVS into the LV throughout the cardiac cycle. This is due to elevated RV pressures in both diastole and systole, flattening the normally circular cross-section of the LV into a ‘D’ shape; this is known as the ‘D-sign’.
A4Ch: There are 4 great signs of RV strain on this view.
- RV dilation with an RV:LV ratio >1 is again seen
- The IVS is seen flattening into the LV
- TAPSE = 11.5mm. This can be appreciated as visually reduced
- McConnell’s sign – Akinesia of the mid-free RV wall with sparing of the apex
SC: The subcostal reaffirms previous findings (although is not the best view to compare chamber sizes). There is no significant pericardial effusion. As the patient breathes, a small round lesion can be visualised in the liver.
*AMVL = Anterior Mitral Valve Leaflet, IVS = InterVentricular Septum, TAPSE = Tricuspid Annular Plane Systolic Excursion
Echo Summary:
- Small LV cavity suggestive of Low LV preload
- RV: RV dilated, IVS flattening into LV, impaired RV longitudinal function (TAPSE), McConnell’s sign, all indicative of RV strain
- Dilated IVC with no respiratory variation, indicative of elevated RAP.
[expandsub1 title=”McConnell’s Sign & RV Strain:” tag=”h3″]
There are a number of echocardiographic signs suggestive of raised pulmonary pressures and ‘RV strain’, of which the most accessible to those learning PoCUS are present above. Echo estimation of pulmonary pressures requires a combination of colour and continuous wave (CW) doppler, and estimation of RAP.
‘McConnell’s’ sign refers to akinesia of the mid-free RV wall with sparing of the apex (seen in the A4Ch view). It was initially described in a cohort with acute PE and was previously thought to be very sensitive and specific for PE. Subsequent studies have demonstrated poor sensitivity, yet in the presence of a high pre-test probability (and especially with a recent normal echo) it may be highly suggestive.
Remember, all the ‘eyeball’ signs for RV strain are all physiological sequelae. In the context of acute PE, this is then by definition a sub-massive PE(see below) and hence carries a worse prognosis than PE without RV strain. This should prompt admission under cardiology for formal echocardiography with estimation* of pulmonary pressures and monitoring with consideration for thrombolysis.
*True measurement of pulmonary pressures requires a pulmonary artery floatation catheter which is rarely performed.
[/expandsub1]
[expandsub2 title=”Thrombolysis in Sub-massive PE – a question rather than an answer:” tag=”h3″]
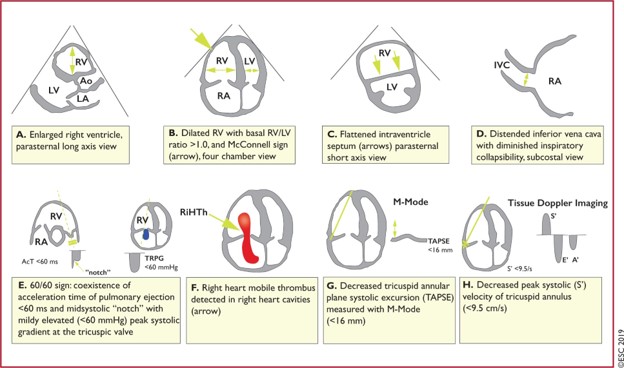
There is significant debate on the role of thrombolysis in sub-massive PE and since this encompasses such a large spectrum, identifying appropriate treatment strategies to all patients across this spectrum is a significant challenge. The evidence is not currently conclusive and definitions/management guidelines differ significantly[3,4]. There is an interesting article by Josh Farkas on this at https://emcrit.org/ibcc/pe/. The AHA classification of PE is listed below, followed by an ESC table on echocardiographic features in RV strain[4].
| AHA Classification of Acute Pulmonary Embolism[3] | |
| Massive | Sustained hypotension (systolic BP <90 mm Hg for 15 min or requiring ionotropic support)-or-Pulselessness-or-Sustained heart rate < 40 BPM with signs/symptoms of shock |
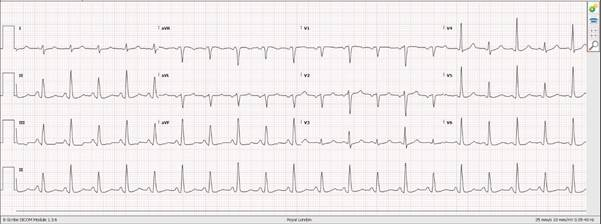
| Submassive | Systolic BP > 90 mm Hg and RV dysfunction or myocardial necrosis defined by:RV dilation (apical 4-chamber RV diameter divided by LV diameter > 0.9) or RV systolic dysfunction on echocardiography-or-RV dilation (4-chamber RV diameter divided by LV diameter > 0.9) on CT-or-Elevation of BNP (> 90 pg/mL), or N-terminal pro-BNP (> 500 pg/mL)-or-EKG changes (new complete or incomplete right bundle-branch block, anteroseptal ST elevation or depression, or anteroseptal T-wave inversion)-or-Elevation of troponin I (> 0.4 ng/mL) or troponin T (> 0.1 ng/mL) |
| Low risk | Absence of the markers of adverse prognosis that define massive or submassive PE |
[/expandsub2]
[expandsub3 title=”What is the most Sensitive & Specific Echo finding for Massive/Sub-massive Pulmonary Embolism?” tag=”h3″]
Echo is can confirm the diagnosis of PE only if right heart thrombi are visualised[4], and this carries a very high early-mortality. The ‘60/60 sign’ and McConnell’s sign have reasonable PPV but are not pathognomonic. After visualised thrombi in situ, an RV:LV diameter ratio >1 and TAPSE <16mm are most associated with poor outcomes[4].
There is another echo assessment that is shows promise in this area and is extremely sensitive for pre-capillary pulmonary hypertension (as found in PE) – ‘Early Systolic Notching’ (ESN). This requires significant echo experience and the study demonstrating this is well described here at UltrasoundGEL. Watch this space.
[/expandsub3]
[expandsub4 title=”Case Progression:” tag=”h3″]
The decision was made not to thrombolyse since the patient was haemodynamically stable and was oxygenating ok on high-flow oxygen. Additionally, CTPA showed possible hepatic lesions and the benefit of thrombolysis in the presence of possible disseminated malignancy was not felt to outweigh risk. A formal CT Abdo/Pelvis showed a pancreatic mass with hepatic dissemination and he was admitted under the medical team. He improved with LMWH and his oxygen requirement reduced over the next few days.
Formal Echo Conclusion:
- There is significant septal compression throughout the cardiac cycle, likely the cause of a very small LV cavity, due to both pressure and volume overload. The LV contractility is dynamic.
- Severely dilated RV with severe impairment
- TR seen with estimated PASp of 56mmHg+RAP, estimated at 15mmHg. [Therefore PASp 71mmHg!!]
Learning Points:
-Echo cannot be used to rule out PE nor rule in (unless clot-in-transit is seen)
-Echo signs of RV strain include RV:LV >1, D-sign, TAPSE<17mm, McConnell’s sign and in the context of confirmed PE are suggestive of submassive PE hence a worse prognosis.
-Management of submassive PE remains controversial as it is a heterogeneous spectrum of patients and evidence for optimal management is still lacking. Management should take into account the patient’s trajectory and local guidelines.
[/expandsub4]
[/expand]
References:
- Dwyer KH, Rempell JS, Stone MB. Diagnosing centrally located pulmonary embolisms in the emergency department using point-of-care ultrasound. (2018) The American journal of emergency medicine. 36 (7): 1145-1150. doi:10.1016/j.ajem.2017.11.033
- Walsh BM, Moore CL. McConnell’s Sign Is Not Specific for Pulmonary Embolism: Case Report and Review of the Literature. (2015) The Journal of emergency medicine. 49 (3): 301-4. doi:10.1016/j.jemermed.2014.12.089
- Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011; 123:1788–1830.
- Stavros V Konstantinides, Guy Meyer, Cecilia Becattini, Héctor Bueno, Geert-Jan Geersing, Veli-Pekka Harjola, Menno V Huisman, Marc Humbert, Catriona Sian Jennings, David Jiménez, Nils Kucher, Irene Marthe Lang, Mareike Lankeit, Roberto Lorusso, Lucia Mazzolai, Nicolas Meneveau, Fionnuala Ní Áinle, Paolo Prandoni, Piotr Pruszczyk, Marc Righini, Adam Torbicki, Eric Van Belle, José Luis Zamorano, ESC Scientific Document Group, 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC),European Heart Journal, Volume 41, Issue 4, 21 January 2020, Pages 543–603, https://doi.org/10.1093/eurheartj/ehz405
- Rivera-Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, Blais D, Janicke D, Melamed R, Mohrien K, Rozycki E, Ross CB, Klein AJ, Rali P, Teman NR, Yarboro L, Ichinose E, Sharma AM, Bartos JA, Elder M, Keeling B, Palevsky H, Naydenov S, Sen P, Amoroso N, Rodriguez-Lopez JM, Davis GA, Rosovsky R, Rosenfield K, Kabrhel C, Horowitz J, Giri JS, Tapson V, Channick R; PERT Consortium. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019 Jan-Dec;25:1076029619853037. doi: 10.1177/1076029619853037. PMID: 31185730; PMCID: PMC6714903.
- Afonso L, Sood A, Akintoye E, Gorcsan J 3rd, Rehman MU, Kumar K, Javed A, Kottam A, Cardozo S, Singh M, Palla M, Ando T, Adegbala O, Shokr M, Briasoulis A. A Doppler Echocardiographic Pulmonary Flow Marker of Massive or Submassive Acute Pulmonary Embolus. J Am Soc Echocardiogr. 2019 Jul;32(7):799-806. doi: 10.1016/j.echo.2019.03.004. Epub 2019 May 2. PMID: 31056367.
- Michael Prats. Early Systolic Notching for Pulmonary Embolism. Ultrasound G.E.L. Podcast Blog. Published on November 11, 2019. Accessed on July 10, 2021. Available at https://www.ultrasoundgel.org/80