Ultrasound of the Week #035
Thanks to Dr Behnaz Mahmoodian for this case. This week’s topics cover some interesting albeit rare echo findings and there’s a bit of a dive into some physics/physiology for those interested later on!
Case:
A 43 year old male presented to ED with left sided chest pain, shortness of breath and palpitations. He had had previous episodes of chest pain and not before attended the ED. He had a troponin of 180 and was due to start treatment for NSTEMI. However, a bedside Echo was done showing the following:
Findings:
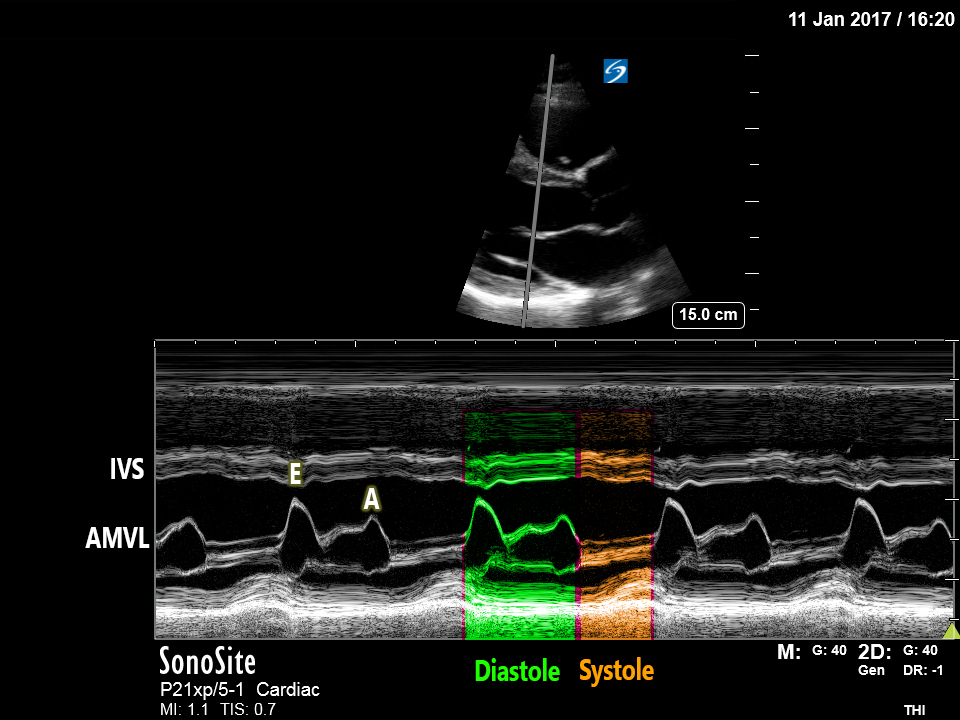
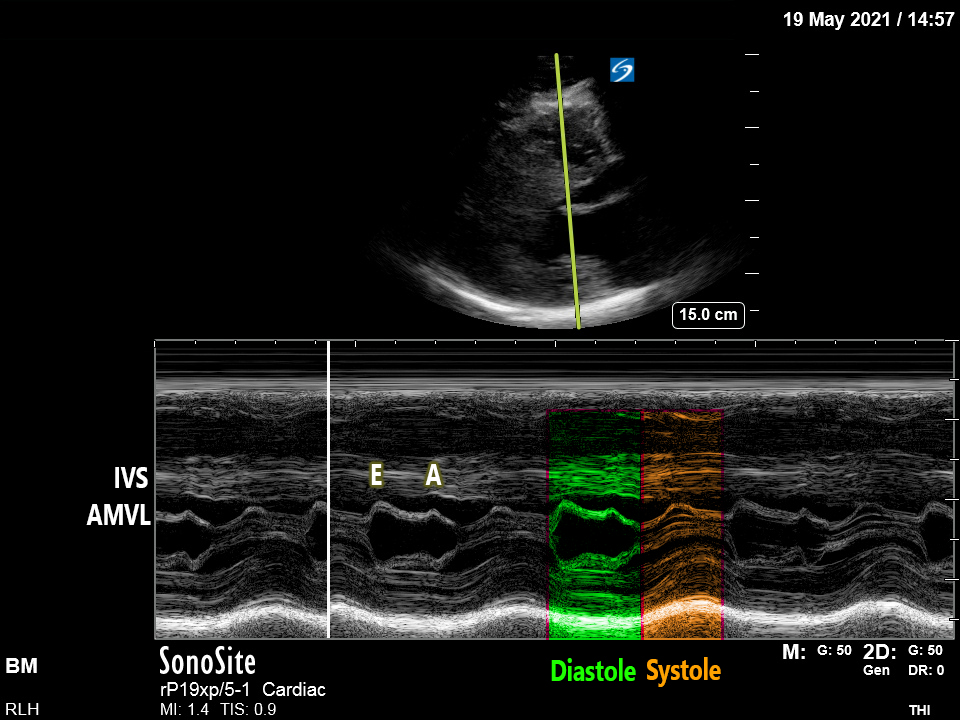
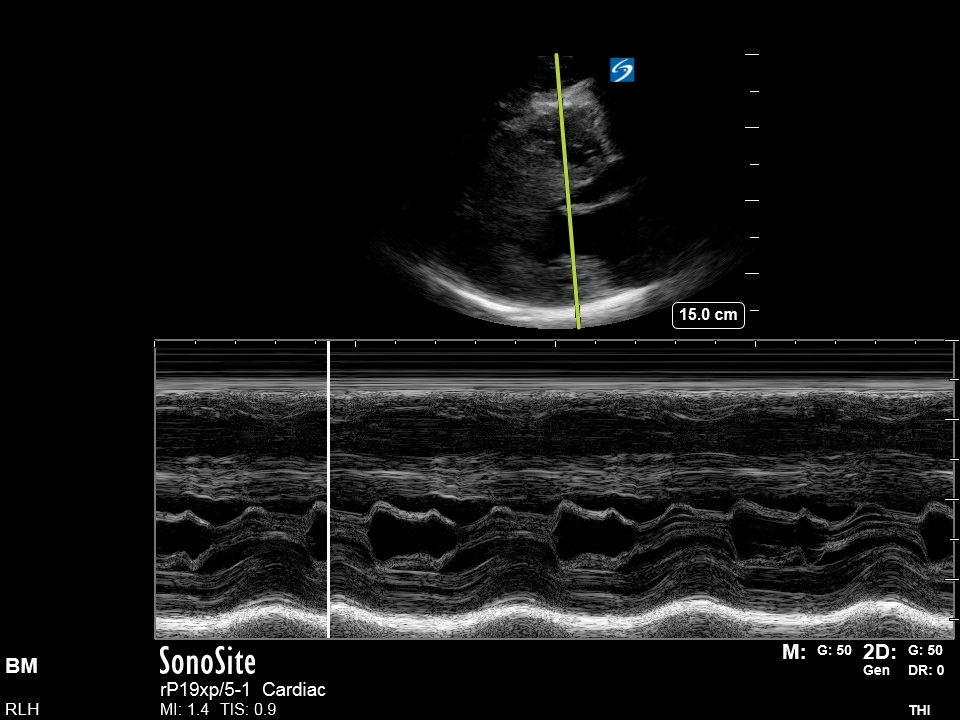
PLAX/PSAX: There is notable LVH with significant septal hypertrophy. The walls appear to touch during systole, obstructing the LVOT. There is a jet of MR visible with colour doppler and M-mode demonstrates Systolic Anterior Motion (‘SAM’) – see below for details.
[expand title=”LV Dimension Measurements in Echo:” tag=”h3″]
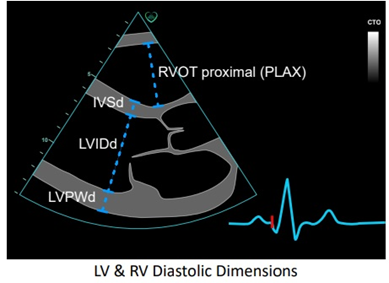
LV wall measurements can be taken in PLAX or PSAX B-mode or M-mode views. Standard measurements are the Interventricular Septum (IVS) and LV Posterior Wall (LVPW), both measured at end-diastole (d).
British Society of Echocardiography (BSE) quote normal measurements as:
- IVS : 6-12mm in males or 5-11mm in females
- LVPW : 6-12mm for males and females.
Measurements in excess of these raise suspicion for LV Hypertrophy. Actual diagnosis of LVH requires LV Mass indexing as per body surface area
[/expand]
[expand title=”Hypertrophic Cardiomyopathy & ‘SAM’ – Description & Management:” tag=”h3″]
Hypertrophic Cardiomyopathy (HCM) is the most common inherited cardiac malformation (around 1 in 500 adults[1]). Around 70% of patients with HCM will have a degree LV Outflow Tract Obstruction (LVOTO), which can be either ‘labile’ (dynamic) or ‘obstructive at rest’, and it is the LVOTO that can cause angina, shortness of breath and sudden cardiac death.
The mechanism of dynamic LVOTO in HOCM involves systolic anterior motion (‘SAM’) of the Anterior Mitral Valve Leaflet (AMVL), which is drawn or ‘pulled’ into the LVOT by the venturi effect**, approaching/abutting the septum and thereby causing both worsening narrowing of the LVOT and posteriorly-directed mitral regurgitation.
Systolic Anterior Motion ‘SAM’:
This is seen best on M-mode assessment of the AMVL[2] in the PLAX view, as used to measure EPSS when estimating LVEF. Look at the comparison of these two images:
[expandsub1 title=”**Some Physics:” tag=”h3″]
For those who’ve made it this far, below is a short description of the Bernoulli principle and Venturi effect, which explain the phenomenon of SAM and hence approaches to treatment.
The Bernoulli principle – explains that to maintain conservation of flow Q, a narrowing of a tube will cause an increase in velocity, and a corresponding drop in pressure, as potential energy (pressure) is converted to kinetic energy (velocity).
Q = A1V1 = A2V2
(Q = flow, A = cross-sectional area, V = velocity)
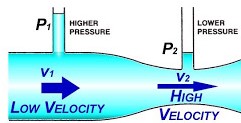
The Venturi effect – if the pressure of a fluid (gas/liquid) is less than that of surrounding other fluid, this fluid will be entrained (‘sucked in’) into the flow. This explains why the AMVL is pulled into the LVOT, causing LVOTO. (This also explains how a venturi mask entrains a known quantity of air, giving the desired FiO2.)
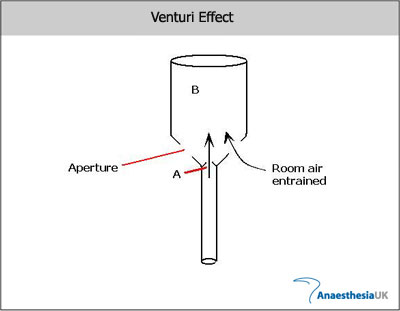
[/expandsub1]
[expandsub2 title=”Management of acute LVOTO:” tag=”h3″]
Woah, too much physics…! ….but this does explain how we can manage dynamic LVOTO in HOCM. The aim is to reduce V2 (thereby entrainment of the AMVL), through increasing LVOT size (“A2”) or reducing Q.
Treatment principles:
- Maintain adequate preload (IV fluids, avoid hypovolaemia/venodilators/diuretics)
- Support afterload – reduces the LV->Aorta pressure gradient, reducing Q, hence velocity V2, reducing SAM (alpha agonists (e.g. phenylephrine), avoid vasodilators)
[/expandsub2]
[/expand]
[expand title=”Case Progression:” tag=”h3″]
Had this gentleman been treated as an NSTEMI with GTN as originally suggested by bloods/ECG, this could have worsened his LVOTO further and therefore been contraindicated. Instead he had a comprehensive echo within a couple of hours confirming moderate LV eccentric hypertrophy, resting LVOTO, with SAM and an eccentric MR jet directed posteriorly.
He was admitted under cardiology and started on a beta-blocker, then discharged with Outpatient Cardiac MRI and ICVD follow up. Bedside echo significantly changed the course of his management and expedited appropriate investigations and treatment.
[/expand]
References:
- Ibrahim Rasmi Ibrahim, MBChB FRCA, Vivek Sharma, MBBS MD FRCA, Cardiomyopathy and anaesthesia, BJA Education, Volume 17, Issue 11, November 2017, Pages 363–369, https://doi.org/10.1093/bjaed/mkx022
- K. Williams, M.P. Frenneaux, R.P. Steeds, Echocardiography in hypertrophic cardiomyopathy diagnosis, prognosis, and role in management, European Journal of Echocardiography, Volume 10, Issue 8, December 2009, Pages iii9–iii14, https://doi.org/10.1093/ejechocard/jep157
- Maron MS. Hypertrophic cardiomyopathy: Clinical manifestations, diagnosis, and evaluation In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on June 04, 2021.)
- LliamEdger, The Physics of fluid flow-a tutorial, AnaesthesiaUK, accessed 04/06/2021 https://www.frca.co.uk/article.aspx?articleid=100482